Adding One Neutron to the Nucleus of an Atom:
The more protons the more love and the electrons are pulled in closer to the nucleus. Thus when 1 neutron is added the atomic mass of the atom increases by 1.

Basic Parts Of The Atom Protons Neutrons Electrons Nucleus Atom Atom Diagram Protons
When you change the number of protons in an atom you will change the atom from one element to a different element.

. Adding a neutron is balanced by adding an electron. The hydrogen atom a single proton and electron has the greatest effect from an added neutron turning it into deuterium. By adding together the number of protons and neutrons and multiplying by 1 amu you can calculate the mass of the atom.
What does adding a neutron to an atoms nucleus do to the atoms mass. So if an atom has equal numbers of electrons and protons the charges cancel each other out and the atom has a neutral charge. D decreases the charge on the nucleus.
Adding one neutron to the nucleus of an atom 1 converts it to an atom of a different element 3 increases its atomic number by one unit but does not change its atomic mass. D increases its atomic number by one unit but does not change its atomic mass. Adding a neutron increases the atoms mass by about 1 AMU assuming it remains stable.
10 Adding one neutron to the nucleus of an atom. The addition of a neutron to the nucleus of an atom. Neutrons play very important role in an atom.
How do neutrons help protons. Click to see full answer. This is because each proton and each neutron weigh one atomic mass unit amu.
Increases its atomic mass by two units but does not change its atomic number. Hence if I have any element lets say Lithium and I add one proton to it you will get Beryllium. Adding one neutron to the nucleus of an atom.
When a neutron is added or removed from the nucleus a new isotope of the same element is formed. The neutron also adds mass to the atom. B decreases the atomic mass of the atom.
Increases its atomic mass by one unit but does not change its atomic number does not change either its atomic number or its atomic mass increases the atom ic number and the mass number by one unit increases its atomic number by one unit but does not change its atomic mass. 4 does not change either its atomic number or its atomic mass. For any given isotope the sum of the numbers of protons and neutrons in the nucleus is called the mass number.
If you add one neutron you have deuterium which is. Your new atom would not actually be an atom if you only add one proton and nothing else. I HOPE THIS HELPED YOU.
C increases the charge on the nucleus. One thing that helps reduce the repulsion between protons within a nucleus is the presence of any neutrons. It adds 1 more unit of mass to the atomic weight of the atom.
The greater binding energy of tritium compared to deuterium shows that the nuclear potential energy does not grow in a simple way with the addition of nucleons the total binding energy is roughly proportional to. You could add a thousand neutrons into the mix and the charge would not change. When you remove or add a neutron to the nucleus of an atom the resulting substance is a new type of the same element and is called an isotopeIf a proton is taken out or added to an atom a whole new element is formed you would also need to add or remove the same number of neutrons to keep the nucleus stable.
A increases the atomic number and the mass number by one unit B increases its atomic mass by one unit but does not change its atomic number C increases its atomic number by one unit but does not change its atomic mass D does not change either its atomic number or its atomic mass. Show activity on this post. What does adding A neutron to an atoms do to the atoms mass.
The number of neutrons in the nucleus of an atom can be determined by 1 adding the atomic number to the mass number 2 subtracting the atomic number from the mass number 3 adding the mass number to the atomic mass 4 subtracting the mass number from the atomic number. The number of positively charged protons in the nucleus of a neutral atom equals the number of circling electrons. They provide stability to the atom and also prevent protons from repelling one another.
The proton number increases by one changing the element of your atom. A neutron in the nucleus doubling the mass. By adding together the number of protons and neutrons and multiplying by 1 amu you can calculate the mass of the atom.
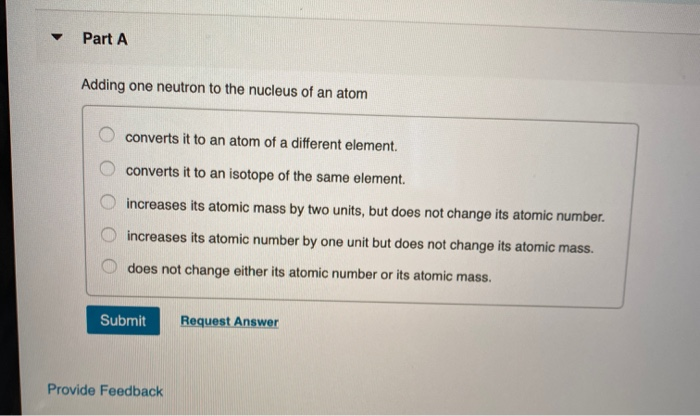
Chemistry questions and answers. The addition of a neutron can make an atom radioactive. Part A Adding one neutron to the nucleus of an atom converts it to an atom of a different element.
In chemical reactions however the nucleus is not involved and nuclear reactions are more a topic in physics. However if you add a thousand neutrons you will be creating one super-radioactive atom. What happens when you add one neutron to the nucleus of an atom.
The only factor that determines the identity of your element is the proton number. C increases its atomic mass by two units but does not change its atomic number. Chemically there are differences in bond energy and length for compounds of heavy hydrogen isotopes compared to normal hydrogen which are larger than the isotopic differences in any.
A increases the atomic mass of the atom. The one I am most familiar with is hydrogen. Unlike the proton the other large subatomic particle that helps form the nucleus of an atom the neutron contains no electric charge.
This is because each proton and each neutron weigh one atomic mass unit amu. B decreases the atomic mass of the atom. B converts it to an atom of a different element.
What is the sum of electron and proton. When are there more protons than electrons in an atom. 2 increases its atomic mass by one unit but does not change its atomic number.
A proton will be added with the neutron increasing the weight by 2 mass units. The neutron is a subatomic particle symbol n or n 0 which has a neutral not positive or negative charge and a mass slightly greater than that of a protonProtons and neutrons constitute the nuclei of atomsSince protons and neutrons behave similarly within the nucleus and each has a mass of approximately one atomic mass unit they are both referred to as nucleons. When tritium is formed by adding a neutron to deuterium 1n 2h æ 3h g a larger amount of energy is released62504 mev.
Converts it to an isotope of the same element. Adding one neutron to the nucleus of an atom A does not change either its atomic number or its atomic mass. Increases its atomic number by one unit but does not change its atomic mass.
If you change the number of neutrons in an atom you get an isotope of the same element. Will a nucleus with more protons or less protons pull the electrons closer. Since they have no charge they dont add to the repulsion already present and they help.
Hydrogen has one proton. If we add or remove neutrons which are not charged we must also add or remove. Neutrons are no exception.
A neutron has no mass so the atomic mass will remain unchanged. The answer is D because the mass of a neutron is taken to be 1 unit. For any given isotope the sum of the numbers of protons and neutrons in the nucleus is called the mass number.
Solved Part A Adding One Neutron To The Nucleus Of An Atom Chegg Com

Proton Particle In Nucleus With Positive Charge Of 1 And An Atomic Mass Number Of 1 Dalton Neutron No Chemistry Education Proton Neutron Electron Electrons
No comments for "Adding One Neutron to the Nucleus of an Atom:"
Post a Comment